
Himani Dhingra 1, Amita Mahajan 2
1Pediatrics, DNB Pediatrics; FNB Pediatric Hematology-Oncology; Attending Consultant Pediatric Hematology- Oncology, Indraprastha Apollo hospital, New Delhi
2Pediatrics, MRCPCH, CCST; Senior Consultant Pediatric Hematology- Oncology, Indraprastha Apollo hospital, New Delhi
*Corresponding Author: Himani Dhingra. Department of Pediatric Hematoncology and BMT, Indraprastha Apollo Hospital, Delhi Mathura Road, Sarita Vihar, New Delhi.
Received: January 02, 2023
Accepted: January 24, 2023
Published: February 27, 2023
Citation: Dhingra H, Mahajan A. (2023). “Ocular Relapse in Acute Myeloid Leukemia”, J Pediatrics and Child Health Issues, 4(1); DOI: http;//doi.org/03.2023/1.1051.
Copyright: © 2023 Himani Dhingra. Alsabawi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Extramedullary relapse (EMR), especially isolated ocular relapse, is rare in childhood Acute Myeloid leukemia (AML) and only a few cases of ocular relapse have been reported. Factors associated with increased risk of EMR are age < 2 years, translocation (8;21), monocytic leukemia and KMT2A gene rearrangements. Ocular relapse may be isolated at diagnosis but is almost always followed by systemic relapse. Optimal treatment is challenging and unclear. Prognosis is extremely guarded. Hereby we report an interesting case of a 20 months old boy undergoing treatment for AML who presented with leukemic hypopyon without previous or concurrent central nervous system (CNS) involvement.
Introduction
Background: Ocular manifestations in leukemia can be present at diagnosis, during treatment or at relapse. Primary leukemic infiltration of the eye can present as orbital infiltration, neuro-ophthalmic signs of CNS leukemia or anterior segment infiltration. Neoplastic hypopyon is a rare manifestation in Acute leukemia and majority of the cases reported to date have been in Acute Lymphoblastic Leukemia (ALL) [1]. Isolated involvement of the AC at relapse is an extremely rare manifestation in AML[2]. We report this unusual manifestation in a young boy undergoing treatment for AML. A written informed consent has been obtained from the family
Case Summary: A 20 months old boy presented to our hospital in July 2021 with a short history of fever, pain abdomen and decreased oral acceptance. The general condition of the child was sick at presentation. On general physical examination there was marked pallor. On systemic examination there was significant abdominal distension with hepatosplenomegaly. On evaluation his Complete Blood count (CBC) showed bicytopenia with initital total leucocyte count (TLC) at diagnosis was 28000/cumm (normal reference range 5000-15000/cumm). Flowcytometry sent from bone marrow (BM) aspirate confirmed the diagnosis of AML with monocytic differentiation i.e FAB M5. Cerebrospinal fluid (CSF) cytology was negative for malignant cells. Next Generation sequencing (NGS) was negative for known pediatric AML mutations.
He was risk stratified as intermediate risk AML as per the ELN (European Leukemia Net) 2017 classification. He was commenced on 3-drug induction chemotherapy as per the ADE regimen (Cytarabine 100 mg/m2/dose twice daily Day 1-7, Doxorubicin 50 mg/m2/day Day 1,3,5, Etoposide 100 mg/m2/day D1-5). He received 2 induction courses with prophylactic triple intrathecal chemotherapy. BM examination at the end of induction (EOI) cycle 1 was in morphological remission and Minimal Residual disease (MRD) measured by 8 colour flowcytometry (FCM) was negative (0.002%). Following this he received 2 cycles of high dose cytarabine 3 gm/m2/dose twice daily Day 1,3,5] (HidAC) consolidation. At the time for admission for the fifth cycle of chemotherapy he was noted to have excessive irritability with redness in the right eye. There was no history of any trauma or other constitutional symptoms. On local examination of the affected eye there was right eyelid edema with conjunctival and circumciliary congestion and significant photophobia but no obvious discharge. He was commenced on broad spectrum antibiotics suspecting an infectious etiology. An ocular oncology review confirmed mild corneal haze and small triangular hypopyon in inferior angle of AC in the right eye. The left eye revealed mild conjunctival congestion with normal AC. Ultrasonography (USG) B scan did not reveal any vitreous exudates in both eyes. Examination under anaesthesia (EUA). confirmed a collection in AC which was aspirated. The fine needle aspiration cytology (FNAC) of the AC fluid confirmed the presence of malignant cells with typical morphology of leukemic blasts (Figure 1).
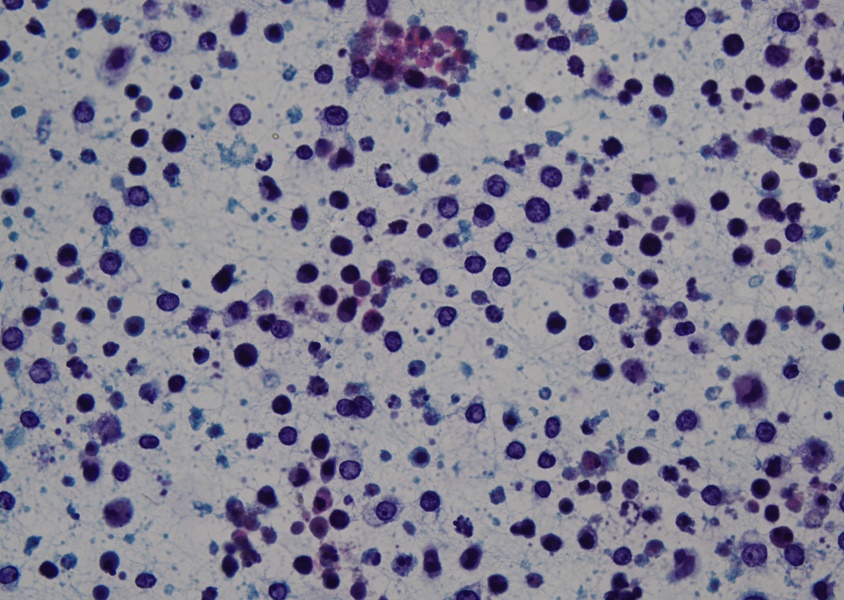
Figure 1: Sheets of atypical round cells (blasts) in a background of histiocytes and mature lymphocytes
He underwent a Magnetic Resonance imaging (MRI) brain with orbit which revealed altered signal intensity of the AC of right globe (Figure 2).
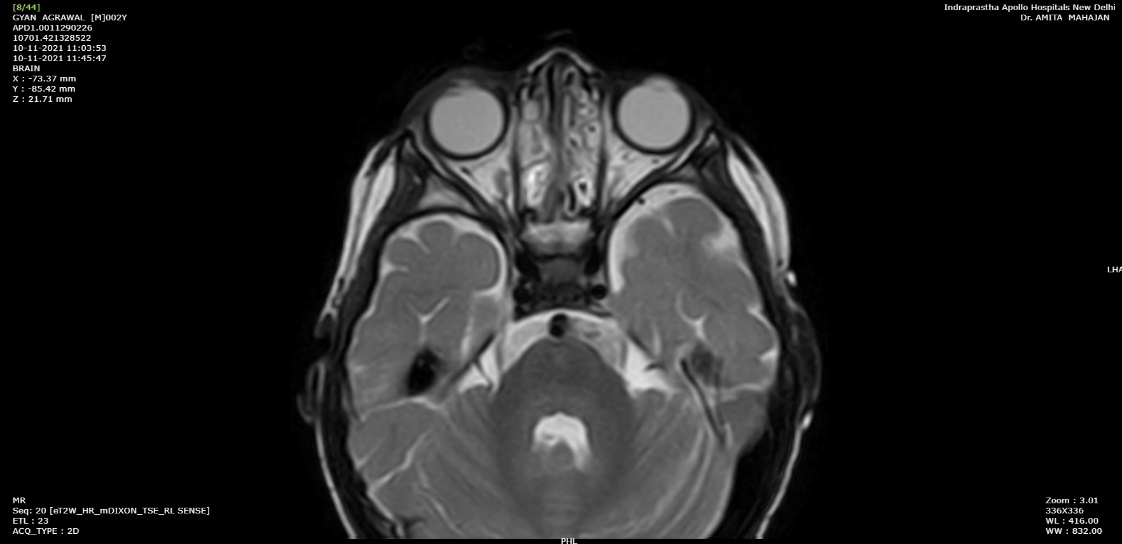
Figure 2: MRI (T2 weighted) showing mild altered signal intensity in anterior chamber of right globe appearing as layering
The lens, posterior chamber and left globe were normal. There was no evidence of leptomeningeal enhancement on imaging. In view of suspected on-treatment relapse he underwent repeat BM MRD and CSF analysis which were negative for disease, confirming an isolated extramedullary ocular relapse.
In view of isolated ocular relapse, it was decided to irradiate this sanctuary site. He received radiation (RT) to whole brain and bilateral (B/L) orbits at a dose 23.4 Gray in 13 fractions. He showed dramatic symptomatic improvement with complete resolution of ocular signs. On reassessment of the eye there was no residual collection in the AC.
Following this he received salvage chemotherapy as per FLAG (Fludarabine 30 mg/m2/day Day 1-5, Cytarabine 2gram/m2/day Day 1-5 with growth factors 5 mcg/kg/day Day 1-7) regimen. Anticipating a higher risk of medullary relapse in future Human Leucocyte Antigen (HLA) typing was done for him and the parents for a possible matched unrelated or haploidentical transplant.
Unfortunately, on day 12 of FLAG chemotherapy his peripheral blood smear showed evidence of atypical cells. FCM confirmed frank hematological relapse. After discussion with family a trial of Venetoclax and Azacytidine was given which yielded no response and he was offered best supportive care. He succumbed to the disease 20 days later.
Discussion:
Ocular manifestations in leukemia can result either from direct infiltration of malignant cells or secondarily due to thrombocytopenia, hyperviscosity or as sequelae to treatment. Ocular manifestations, in general have been reported more oftent in adults [2-5] with retinal haemorrhage being the most common ophthalmic manifestation [3].
Sanctuary sites serve as a reservoir for leukemic cell proliferation and subsequent systemic relapse. The blood-ocular barrier creates a ‘chemotherapy sanctuary’ which prevents the eradication of neoplastic cells from this site. EMR is far more common in ALL than in AML. In a retrospective analysis by Kim J et al [6], CNS and bone have been reported as the most frequent sites of EMR in children with AML. None of the patients were reported to have an ocular relapse. Apart from the retrospective review by Matano et al [1] there has been only few other pediatric case report of AML relapse presenting as leukemic hypopyon [7]. Wesley et al [8] have reported EMR relapse manifesting as a serous retinal detachment in a young teen.
The risk factors predicting EMR have not been consistent across the studies. In the case review of 14 AML patients with leukemic hypopyon, Matano et al [1] identified monocytic leukemia as a possible risk factor to develop leukemic hypopyon. Majority of the patients reported in this review developed the hypopyon while on therapy and presence of CNS disease was strong risk factor. A recent review by Kim J et al [6] found no significant association between extramedullary disease at diagnosis and EMR. AML patients with EMR were reported to have higher incidence of FAB M2 morphology and translocation (8;21). [6]
Favourable cytogenetic and MRD status post induction chemotherapy are considered the two most significant predictors of outcome in AML. Our patient had complete morphological remission and MRD post induction was negative. It is likely that other factors determine the risk of EMR.
Prompt recognition and evaluation of ocular symptoms is mandated in a child with leukemia undergoing treatment or post treatment completion. The symptoms can range from conjunctival injection or discharge, headaches, signs of pre- septal inflammation, decreased visual acuity to loss of vision. These symptoms can be an isolated manifestation of relapse.
As the ocular symptoms are non –specific and mimic many other common eye conditions, the diagnosis may be missed. Though early diagnosis is essential for timely treatment, most of these patients have an eventual systemic relapse. The interval between the EMR and systemic BM relapse can be variable ranging from a few days to 2 years [5]. Kim et al [6] reported a mean interval of 3.4 months in their series.
There is insufficient literature to guide the treatment for isolated ocular relapse of AML due to rarity of this entity. A study by Johnston et al [9] suggested that there was no survival benefit of adding systemic therapy to only organ directed treatment in cases of isolated EMR of pediatric AML. Our patient received cranial + orbital RT anticipating a CNS relapse and FLAG chemotherapy. Unfortunately, he had an overt medullary relapse within 6 weeks of the ocular relapse. Recently there is some evidence for intravitreal chemotherapy for treatment of intraocular leukemia[10]. Further studies are needed to define the risk factors and to guide therapy for EMR in AML.
Long term remissions, despite intensive treatment, are rare. The survival outcomes have been dismal in patients with leukemic hypopyon. Around 60% patients died within 3 months and around 86% died with 1 year of diagnosis of leukemic hypopyon [1]. Patients with EMR with concurrent BMR had grave clinical outcomes [6].
Conclusions: Ocular symptoms in a child with leukemia while on treatment or post treatment completion should be thoroughly investigated. A leukemic relapse should be considered as a differential diagnosis in patients presenting with unexplained anterior ocular symptoms. Cytopathological examination of the anterior chamber fluid is essential to confirm the diagnosis of isolated ocular relapse. Treatment strategies have not been clearly defined due to rarity of EMR in pediatric AML. The prognosis remains guarded despite therapy.
Abbreviations Table:
|
ABBREVIATIONS |
FULL FORMS |
|
EMR |
Extra Medullary Relapse |
|
AML |
Acute Myeloid Leukemia |
|
FAB |
French-American-British |
|
CNS |
Central Nervous system |
|
AC |
Anterior Chamber |
|
ALL |
Acute Lymphoblastic Leukemia |
|
CSF |
Cerebrospinal Fluid |
|
BM |
Bone Marrow |
|
NGS |
Next Generation Sequencing |
|
ELN |
European Leukemia Net |
|
FCM |
Flowcytometry |
|
EOI |
End of induction |
|
Hidac |
High dose cytarabine |
|
MRI |
Magnetic resonance imaging |
Conflicts of interest: None
Funding source: None